PREVIOUS AND CURRENT RESEARCH
Self-assembly of proteins and peptides into amyloid fibrils occurs across all kingdoms of life. The phenomenon is notoriously associated with neurodegenerative and systemic diseases; however, amyloids also play critical roles during microbial infections both as host-derived antimicrobials and microbial virulence factors. The latter has rendered bacterial amyloids as attractive candidates for molecular and structural characterization aimed at understanding self-assembly and discovering novel therapie. In contrast to the vast body of knowledge on eukaryotic amyloids and dysbiosis during neurodegenerative diseases, high-resolution structures and molecular level characterization of the regulation, activity, mechanisms and interactions of microbial amyloids is lacking. The urgency in filling the knowledge gap between eukaryotic and bacterial amyloids has recently been exacerbated by paradigm-shifting Alzheimer’s disease research, including the antimicrobial protection hypothesis.
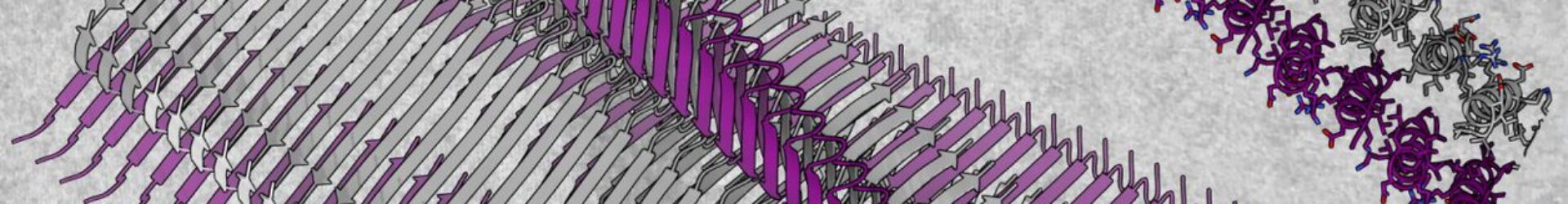
A novel cross-α amyloid form.
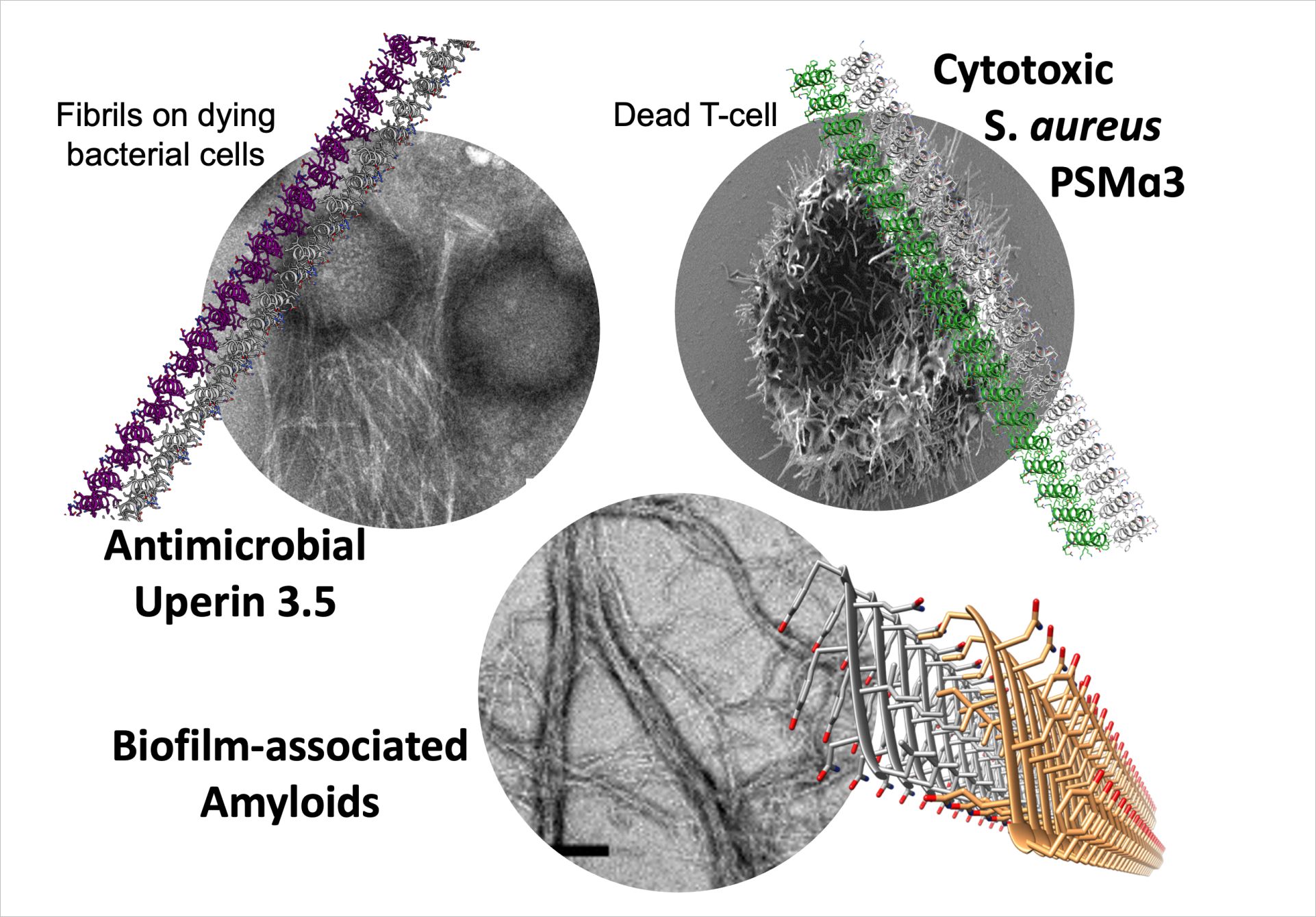
While human amyloids share cross-b features of tightly mated b-sheets, the Landau group discovered a novel amyloid form that is composed entirely of amphipathic α-helices that stack perpendicular to the fibril axis into mated cross-α ‘sheets’ (Fig. 1) (Tayeb-Fligelman et al., Science 2017; ref 1). The cross-α fibril form was revealed by the crystal structure of the cytotoxic phenol-soluble modulin α3 (PSMα3) peptide secreted by the pathogenic Staphylococcus aureus bacterium. This also provided the first high resolution structure of a bacterial amyloid fibril. We showed that cross-α fibrillation facilitated PSMα3 cytotoxicity against human T2-cells. Extensive mutagenesis analyses suggested that PSMα3 cytotoxicity involves a dynamic process of co-aggregation with the cell membrane, and subsequent cell rupture [refs 1,2,6].
The cross-α fibril form was shown once more by the crystal structure of the uperin 3.5 antimicrobial peptide (AMP) secreted by Uperoleia mjobergii (Mjoberg’s toadlet) (Salinas et al., PNAS 2021; ref 8). Surprisingly, uperin 3.5 showed a secondary structure switch between cross-a and cross-b fibrils, depending on environmental conditions. In particular, the presence of lipids which drive a transition to an α-helical state. Therefore, as evidence mounts to suggest that the diversity of fibril morphological space is correlated to activity and controlled by environmental cues, we need to expand the catalogue of amyloids structures.
The amyloid-antimicrobial link.
A potential physiological link between amyloid production in humans and resistance to microbial infections was previously suggested by Tanzi, Moir and others. The uperin 3.5 atomic resolution data revealed a link between a-helical amyloid fibrils and antimicrobial activities by showing the first known antimicrobial peptide with a capacity to form both cross-α and conventional cross-β amyloid fibrils. Altogether, these studies complement previous work on neurodegenerative-associated amyloids displaying antimicrobial activity.
FUTURE PROJECTS AND GOALS
Our findings and the advent of novel imaging techniques, including cutting-edge approaches of cryogenic electron light and correlative microscopy and tomography, now allow us to pose our ambitious overarching goal.
- We aim to elucidate the specific relationships between membrane interaction, fibril formation, secondary-structure, morphology, and activities of virulent and antimicrobial amyloids.
- A cross α/β switch regulation model will be evaluated via structural and biophysical studies, mutations and truncations, and visualization of the interactions with membrane mimetics and cells.
- We aim to identify more general features that regulate the fibril secondary structure switch and its functional implications, and use this information for the design of novel antimicrobial agents.
FUNDING






Current Funding:
We are grateful for funding from the Helmholtz Association (Impulse and Networking Fund), the European Union (ERC, FuncAmyloid), Cure Alzheimer’s Fund, PIER seed funding and HALRIC (Interreg).
Past Funding @Technion:
We are grateful for funding from the Technion, Israel Science Foundation – Individual Research Grant, U.S.-Israel Binational Science Foundation (BSF), Forschungskooperation Niedersachsen – Israel (Research Cooperation Lower Saxony – Israel), Volkswagenstiftung, from the Israel Ministry of Science, Technology and Space, DIP – Deutsch-Israelische Projektkooperation, University of Michigan – Israel Collaborative Research Grant, Henri Gutwirth Fund for the Promotion of Research, Israel Science Foundation – Individual Research Grant, U.S.-Israel Binational Science Foundation (BSF), Marie Curie Reintegration Grant, the I-CORE Program of the Planning and Budgeting Committee and The Israel Science Foundation (I-CORE Center of Excellence in Integrated Structural Cell Biology), the Henry Taub Prize for Academic Excellence. Umbrella Cooperation – When Life Sciences and Engineering Converge, Eliyahu Pen research Fund, Mallat Family Research Fund, J. and A. Taub Biological Research, and Alon Fellowship from the Israeli Council for Higher Education.